May 2017 | Point of View
A globalized model for medical affairs
A globalized model for medical affairs
Pharmaceutical companies that have adopted a patient-centric approach have seen significant benefits in the form of greater patient adherence to medication, more consistent customer engagement, and overall improvements in the efficiency of their medical information and pharmacovigilance operations. Through its critical role in communicating with patients and other stakeholders, the Medical Affairs function is at the center of the industry’s move toward patient centricity—yet the function’s current regional orientation could be at odds with this goal.
This white paper outlines three steps that pharmaceutical Medical Affairs functions should take to support a robust patient experience strategy and transform current operations into a global, integrated model.
Breaking down the silos to improve patient experience and quality of care
In recent years, pharmaceutical companies have begun to embrace a patient-centric business model—one that views an individual who uses a drug product as a person on a treatment journey rather than a data point in a population set. This model originated in the healthcare sector, and the pharmaceutical industry has since customized and evolved it to suit its own needs.
The Medical Affairs department is at the front line of evolving an organization’s degree of patient centricity. However, the department’s mission to fulfill the critical role of answering questions about drug interactions and product efficacy is often complicated by:
- Broader expectations around building and sustaining product knowledge bases
- Maintaining information in multiple languages
- Staying abreast of regulations worldwide
- Maintaining total visibility for product labeling
- Facilitating internal and external collaboration
- Identifying and managing relationships with industry opinion leaders
- Producing informed opinions about the best collaboration tools.
Performing all these tasks well is a tall order. Often, the department’s structure inhibits its ability to fulfill these critical missions.
Historically, pharmaceutical companies have organized the Medical Affairs department regionally instead of globally by therapeutic area. While this structure may be closer to constituents, it can produce substantial regional differences in how organizations communicate medical information to patients and other stakeholders, potentially affecting the patient and stakeholder experience. Moreover, for therapies that generate hundreds of thousands of inquiries each year, regionally organized Medical Affairs departments and response libraries can be highly inefficient due to redundant activity and duplicative investments in call centers and personnel.
Given all this, it is not surprising that most Medical Affairs knowledge resides in silos. When this is the case, it can inhibit an organization’s ability to become more patient-centric.
Facilitating the journey to a patient-centric model
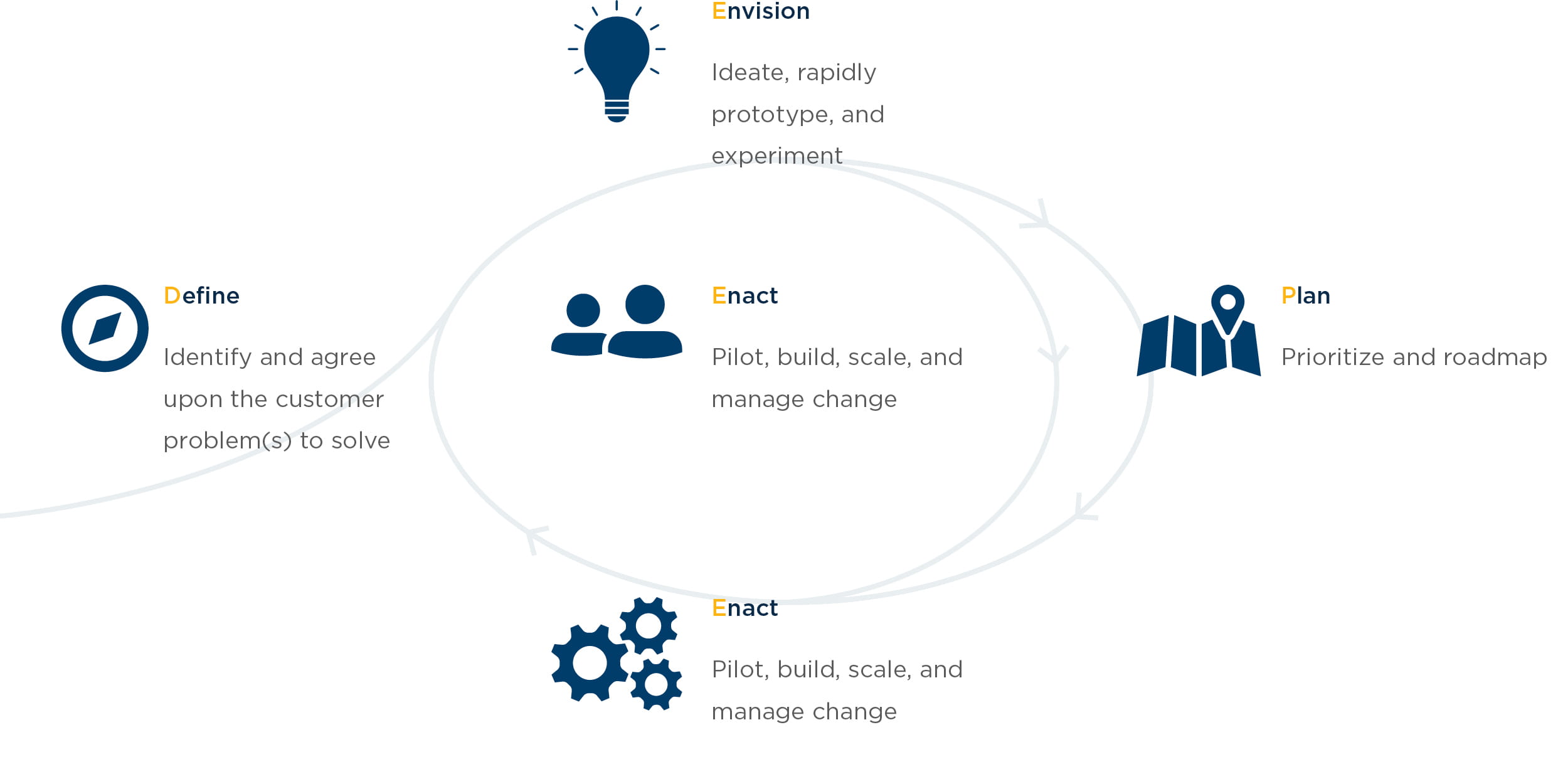
West Monroe uses a practical DEEPEN (Define, Empathize, Envision, Plan, Enact) framework to develop patient experience strategies, including strategies that drive more effective Medical Affairs functions.
This type of framework facilitates organizational alignment and creates a roadmap for initiatives that enhance patient experience by:
- Establishing a “North Star” vision for the patient experience
- Gathering insights and research that provide a pulse on patient health, attitudes, and behavior
- Building a roadmap for delivering on the vision
From this framework, we identified three critical steps for advancing patient centricity within Medical Affairs.
1. Evolve Medical Affairs operations from a regional model to a global, integrated model
The industry is shifting toward a centralized model for medical knowledge sharing that is organized by therapeutic area rather than region. Such models provide a consistent approach for medical information responses, case management, and case resolution. At the same time, they also consider and account for regional differences in regulatory requirements and languages.
In addition to improving the patient experience, global Medical Affairs processes and systems can reduce costs significantly and improve operational efficiency by limiting duplicative information development and case management efforts, reducing reliance on key opinion leaders and external experts, and decreasing the footprint of regional IT systems and call centers.
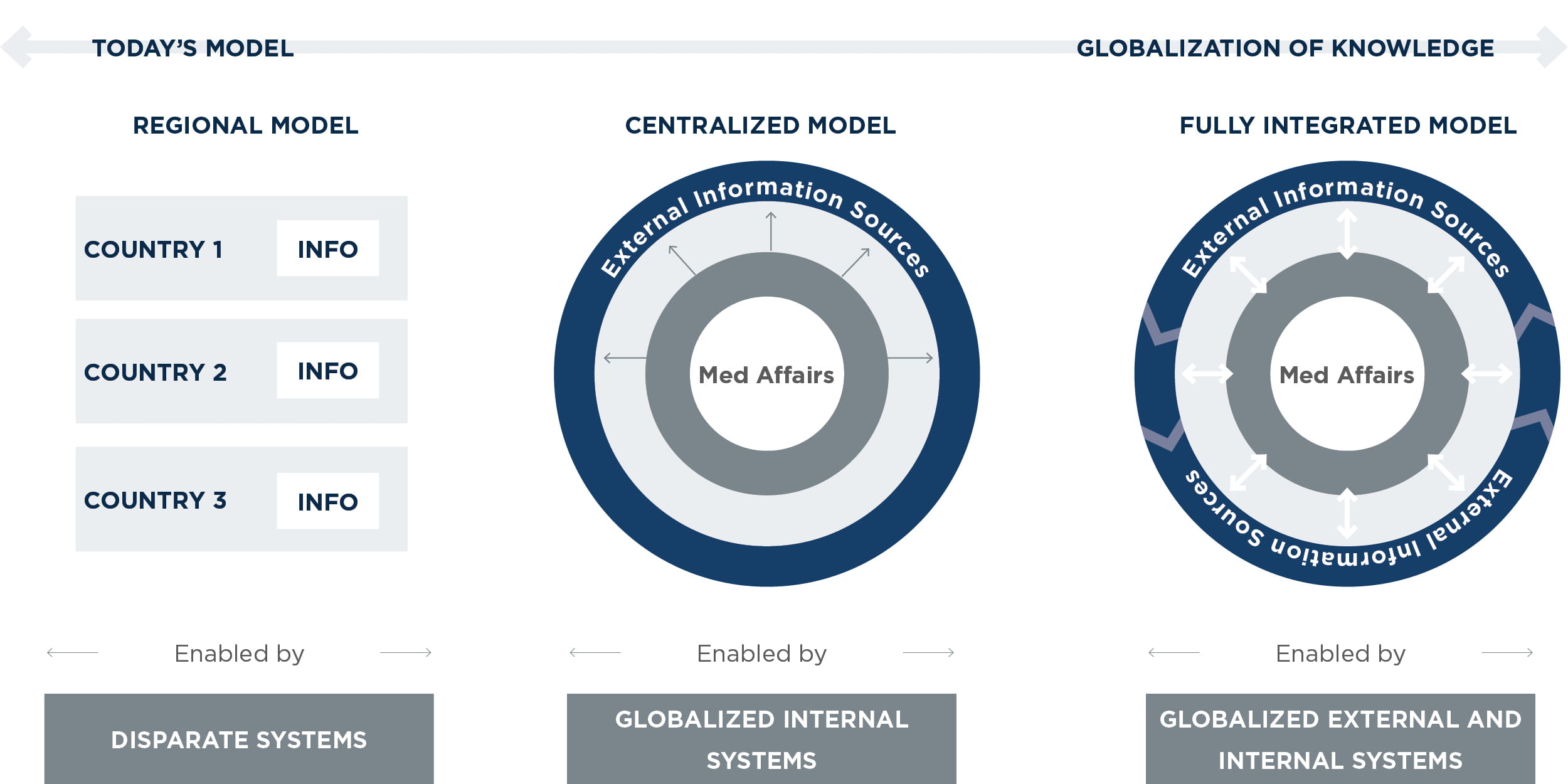
Although the shift from a regional model to a global model represents a significant change, it is possible to stage the necessary changes in people, processes, and technology over a three- to five-year period. That shift may look like the following:
Current regional model: In the current regional model, each region produces its own medical responses and manages its own case management processes, external collaboration initiatives, and IT systems. As a result, responses to patients, payers, regulatory agencies, and others may be inconsistent or duplicative.
Centralized model: This model addresses the first hurdle to globalization by collecting all country data in a centralized, integrated system. This includes a central repository of responses and cases. Knowledge management becomes more standardized and less “tribal” as the organization deploys new global capabilities such as enterprise search, collaboration platforms, medical information systems, and master data management. (See sidebar, “Powerful search”) Some regional silos may continue to exist to address specific regulatory requirements, and there may be duplicative interaction with external key opinion leaders and patient advocacy groups.
Fully integrated model: This model has fully integrated knowledge management systems and processes that enable the flow and exchange of information among internal and external medical stakeholders globally and across all areas of the business. It benefits from capabilities such as external collaboration platforms, artificial intelligence, and external data ontologies.
2. Understand and engage key medical information stakeholders
The first step to improving the patient experience is to understand the key stakeholders, their roles, and how they interact with the organization. This involves studying medical information business processes, the stakeholders involved in those processes in various countries, common types of inquiries and how those originate, common types of responses to those inquiries, and regulatory requirements. For a large global pharmaceutical organization, this can be complex—medical information can originate from many different stakeholders, and processes can vary significantly depending on where or from whom an inquiry originates. Moreover, the organization may have varying goals with respect to each stakeholder group.
Given the diversity of stakeholders, it is important to understand how collaboration with these constituents affects key business processes and ultimately the patient experience. Patient journey mapping can illustrate these impacts and identify pain points that represent opportunities for improving and centralizing Medical Affairs operations while enabling stakeholders to engage more effectively with patients.
From this analysis, a pharma company can refine its strategies for engaging with and providing information to patients and stakeholders. This may include:
- Developing communication and data-sharing tools and processes, such as centralized procedures for responding to inquiries (See the sidebar, “Standardizing responses to inquiries to deliver a consistent experience”)
- Centralizing medical information used by the global organization
- Delivering timely and accurate drug information and compliance processes to all stakeholders
3. Implement a tactical IT strategy with new tools and technology that collect and track data about patient/stakeholder interactions
Most organizations already own and have deployed an array of technology solutions that touch stakeholders and patients. Establishing truly patient-centric services, however, will require a fresh look at IT strategy once the organization has identified its key stakeholders and mapped the patient journey.
Various tools and systems can help Medical Affairs functions collect and track interaction data and improve patient centricity. These include:
Citation analysis mining via machine learning to extract relevant insights. Use machine learning to automate research citation analysis facilitation and integration of public global health data, third-party licensed data, and internal data into a single, holistic view. This allows Medical Affairs to identify new, upcoming key opinion leaders by therapeutic area. Robust visualization tools then use the output data to identify correlations through development of heat maps and association trees.
Automated, personalized dashboards with natural language generation. Artificial intelligence allows organizations to assess, combine, and then summarize large amounts of content in the form of narratives that mimic human written language. Dashboards with both data and narrative content can be automated and refreshed as data sets update. Because this type of communication is from machine to machine, such narratives largely meet CFR-Part 11 compliance requirements.
Elevating the role of Medical Affairs. These tools transform knowledge into capabilities that can be used across the organization for predictive purposes, allowing Medical Affairs to elevate its role as curator and knowledge capital broker.
When adopting any new technology, it is prudent to use a well-established software selection approach that is based on stakeholder needs and business requirements.
Walking the talk: How to shift toward patient-centricity
A global, integrated model delivers substantial benefits in the form of lower costs and greater efficiency. For example, a global model enables Medical Affairs to:
-
Reduce the time required to look for documents and knowledge assets or to send requests for information
-
Minimize the need to repurchase content by making previously completed studies, publications, and medical content more accessible
-
Eliminate duplicative effort across regions
-
Enhance collaboration and team interactions to stimulate innovation
More significantly, by elevating patient centricity, it can potentially improve every segment of the healthcare system:
-
For patients, it enables higher quality care, leading to better health and greater satisfaction with the system
-
For providers, it creates a high-quality, safer delivery environment with more productive employees—factors that can drive higher revenue
-
For payers, it can reduce costs and lower premiums through more efficient interactions with patients and providers
-
For pharmaceutical companies, it provides greater insight into patient behavior, allowing organizations to create innovative solutions that attract, engage, and retain patients
Capturing insights about today’s internal and external stakeholder interactions is a key first step in the transformation to a patient-centric, global Medical Affairs operation. Although this can be a challenging path, with all stakeholders contributing, a patient-centric model will facilitate better quality care at a lower cost.